About Us
Our company is the pioneer in meeting the needs in the field of radiology in Turkey started its activities in 1988 and is the center of a group of companies in the Middle East, stretching from Europe to America and then to Asia.
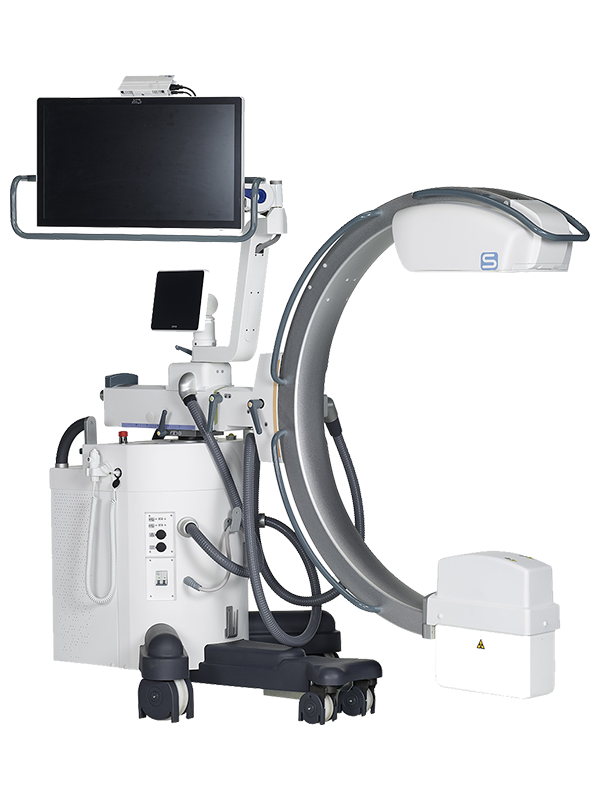
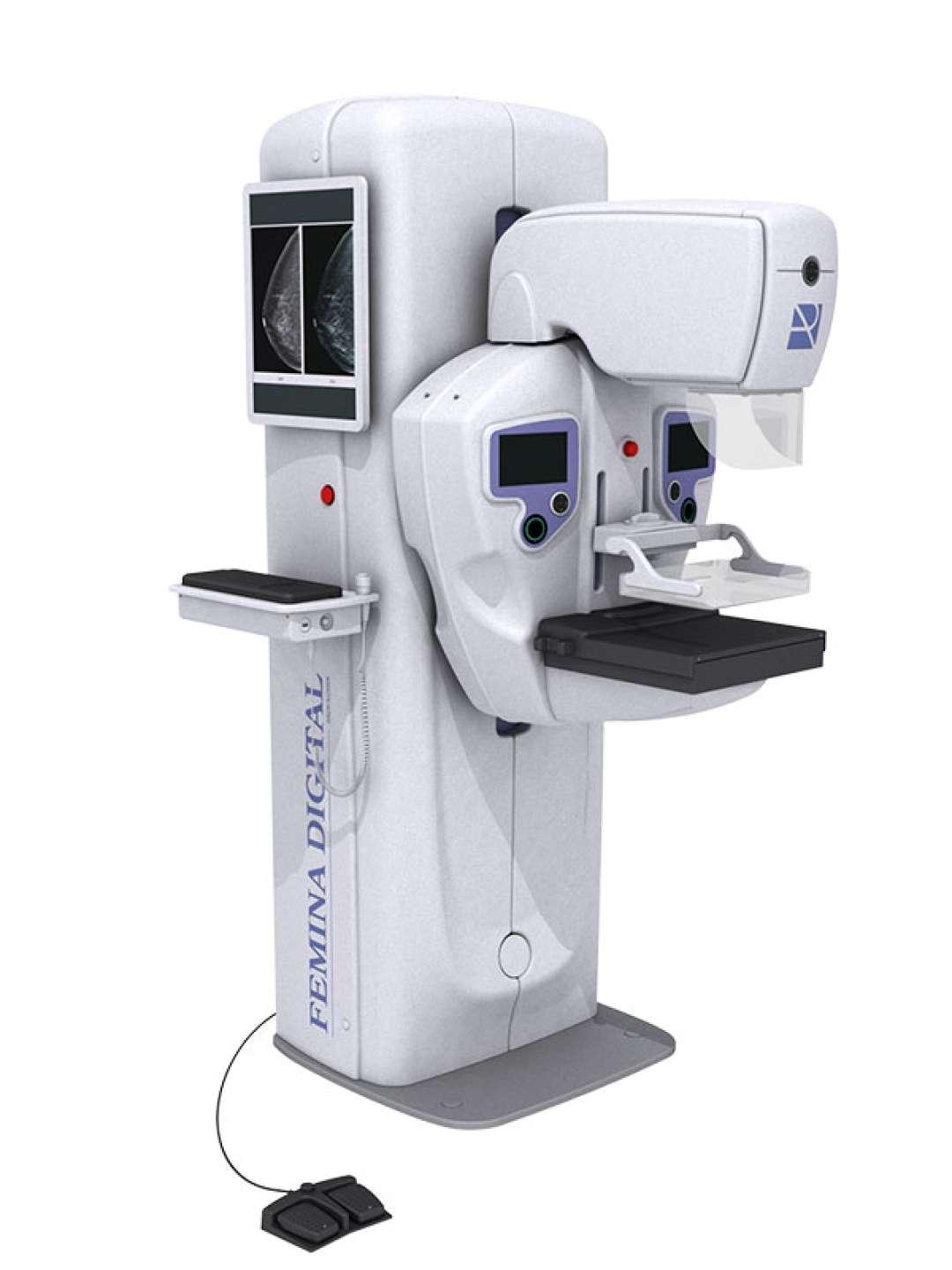
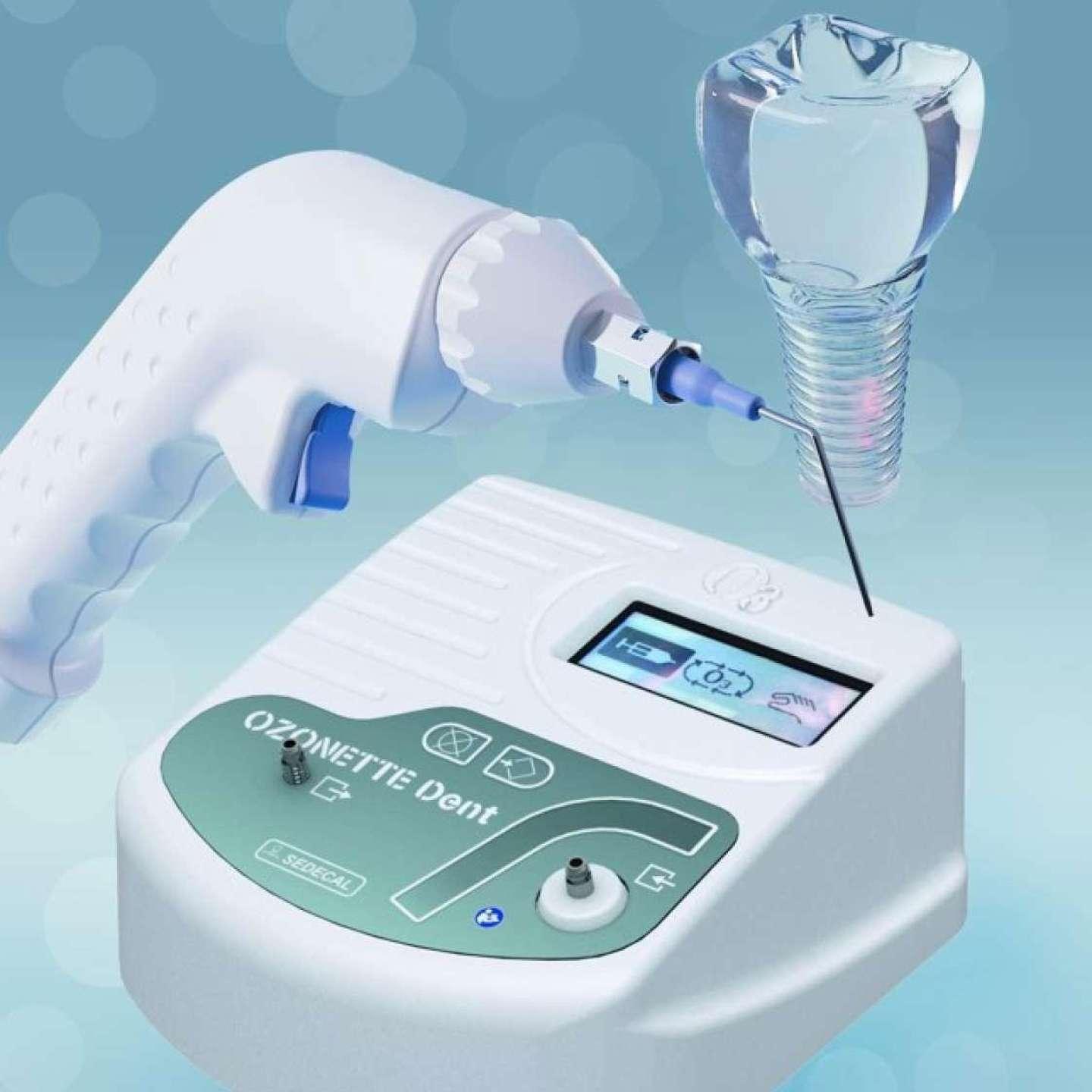
We have been serving hospitals, clinics and imaging centers in the field of radiology for more than 35 years with our diversified range of products such as digital radiology systems, mobile x-ray units, surgical C-Arm, mammography, digital fluoroscopy tables, veterinary imaging systems and ozone generators.
USX-RAY röntgen A.Ş's activities in the field of radiology are not limited to the services provided to the end user but also include increasing the export volume in the progress of time with the productions it carries out for its foreign stakeholders. With this aspect, our company distinguishes itself from other companies in the sector, contributes to the production of imaging systems and is a pioneer in this field in our country. Today, the countries that make up the export network of USX-RAY Röntgen A.Ş are mainly; Spain, France, Italy, Hungary and Canada. Our knowledge and experience are recognized countrywide with more than 3000 imaging systems installed in the field.
Our company, which offers strong after-sales service support to our customers with our knowledge and experience, has achieved to certify its activities since 2005, which it has carried out with the awareness of serving the health sector and it has made a point of keeping up to date.
US-X RAY RÖNTGEN A.Ş. Quality Policy
Our policy is to perform production, assembly, service and all activities of radiographic imagining systems in accordance with the customer demands and satisfaction, by applying ISO 9001 and ISO 13485 quality systems and following the regulatory requirements of MDD 93/42/EEC & 2007/47/EC and Regulation EU 2017/745 by continuous improvement of its products and processes, by following the technological developments, by intending the leader position in the country, by increasing its market share in the world, performing production and servicing with the conscience of offer ing service to the human health."

ISO 9001 certification is a voluntary certification of the quality management system based mainly on the improvement of customer satisfaction and its processes.

ISO 13485 certification is a voluntary quality management system certification for medical device companies to meet applicable regulatory requirements.

CE Marking indicates that your medical device complies with applicable EU regulations, and enables the commercialization of your products across all EU member states.

The CSA mark ensures that USX-RAY export products meet American and Canadian standards.